Sustainability: The Direct Result of Responsible Leadership
The Bracco approach to conserve iodine, reduce waste, lower costs
Bracco led the way in the industry by partnering with the FDA: Together we developed the IBP solution to help resolve workflow/resource challenges in the CT suite1
Using IBP is a compliant way to help:
- reduce costs
- waste less contrast
- save time
- individualize doses
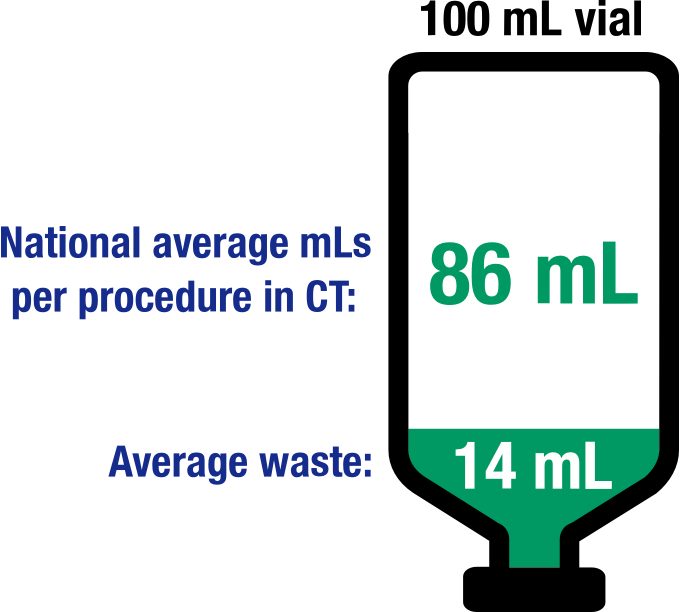
Individualized dosing can reduce contrast waste and cost2

Personalized dosing using IBP is a recommended strategy for conserving iodine3
The Bracco approach to conserve iodine, reduce waste, lower costs
Bracco led the way in the industry by partnering with the FDA: Together we developed the IBP solution to help resolve workflow/resource challenges in the CT suite1
Using IBP is a compliant way to help:
- reduce costs
- waste less contrast
- save time
- individualize doses
Individualized dosing can reduce contrast waste and cost2
Eliminate Waste From Single-Use Containers
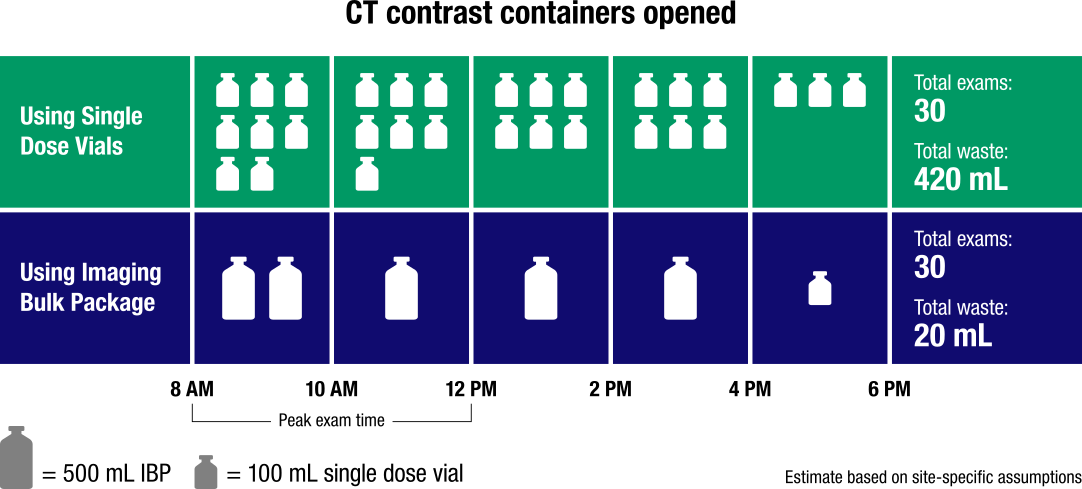
ISOVUE Imaging Bulk Package saves contrast and disposal costs associated with bottles4

Established Safety
ISOVUE products provide established efficacy and safety, 3,000+ published studies, and 40+ years of trusted use
Increased Efficiency
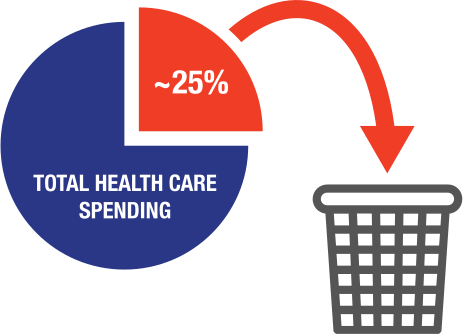
Approximately 25% of total health care spending can be attributed to waste.5 Let us help you create the right intervention at the right time, for each patient.
Greater dosing efficiency results in less waste and improved cost savings1
- Accommodates multiple patients, procedures, and protocols6
- Streamlines processes by reducing time required for equipment setup
- More efficient than single-use vials and pre-filled syringes
- Simplified purchasing and inventory
- Fewer vials = less medical waste
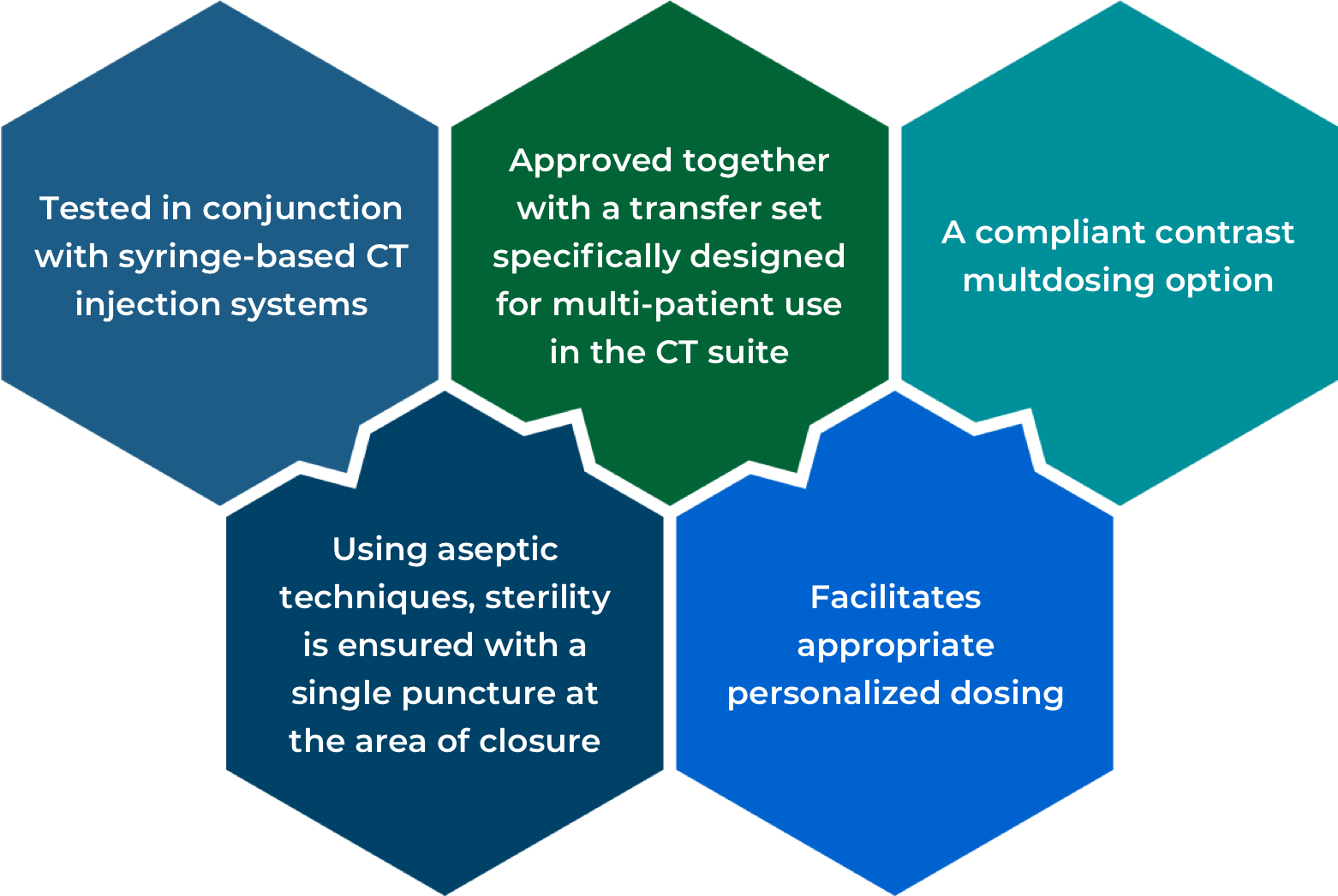
IBP Product Features
Maximum use time
ISOVUE Imaging Bulk Package provides convenient dosing from the same container up to 10 hours after initial puncture to accommodate multiple patients.6*
*A maximum use time of 10 hours from initial closure entry is permitted to complete fluid transfer.
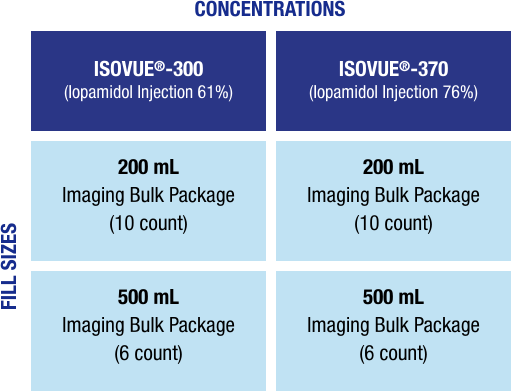
Flexible packaging options save time
Also available for cardiac catheterization

We’re waiting for your questions
Want to get in touch with us?
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
PRODUCT NAME®
IMPORTANT SAFETY INFORMATION
WARNING: Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Praesent mattis eget nunc elementum malesuada. In et nunc lobortis massa maximus ullamcorper nec quis ante. Integer et dolor turpis. Vivamus id semper odio. Integer venenatis viverra elit vel interdum. Nam lectus diam, dictum non erat at, rutrum faucibus tellus. Proin in nulla sagittis, mollis eros eu, iaculis tortor. Nulla non sem scelerisque, congue ante vitae, venenatis urna. Phasellus et viverra leo. Nunc at ultricies felis. Nulla suscipit pulvinar euismod. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Nunc eu erat tincidunt, lacinia arcu eu, maximus lectus.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
PRODUCT NAME®
IMPORTANT SAFETY INFORMATION
WARNING: Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Praesent mattis eget nunc elementum malesuada. In et nunc lobortis massa maximus ullamcorper nec quis ante. Integer et dolor turpis. Vivamus id semper odio. Integer venenatis viverra elit vel interdum. Nam lectus diam, dictum non erat at, rutrum faucibus tellus. Proin in nulla sagittis, mollis eros eu, iaculis tortor. Nulla non sem scelerisque, congue ante vitae, venenatis urna. Phasellus et viverra leo. Nunc at ultricies felis. Nulla suscipit pulvinar euismod. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Nunc eu erat tincidunt, lacinia arcu eu, maximus lectus.
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes administration
- Always have resuscitation equipment and trained personnel readily available
Contraindications
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is contraindicated in patients with known or suspected hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG).
Warnings
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including LUMASON. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to LUMASON administration and monitor all patients for acute reactions.
Post-marketing hypersensitivity reactions, including serious hypersensitivity reactions, have been observed during use or shortly following LUMASON administration. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. LUMASON contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG.
Systemic embolization may occur in patients with cardiac shunts. Assess patients with cardiac shunts for embolic phenomena following LUMASON administration.
There is a risk of ventricular arrhythmia related to high mechanical index in patients administered LUMASON. LUMASON is not recommended for use at mechanical indices greater than 0.8.
The most common adverse reactions (incidence ≥ 0.5%) are headache (1%) and nausea (0.5%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for LUMASON ultrasound contrast agent, including BOXED WARNING on Serious Cardiopulmonary Reactions.
LUMASON is manufactured for Bracco Diagnostics Inc., Monroe Township, NJ 08831 by Bracco Suisse S.A., Plan-les-Ouates Geneve, Switzerland (LUMASON lyophilized powder vial-25 mg lipid-type A/60.7 sulfur hexafluoride gas); Vetter Pharma-Fertigung GmbH & Co. KG, 88212 Ravensburg, Germany (Sodium Chloride 0.9% Injection, USP); B. Braun Melsungen AG, 34212 Melsungen, Germany (Mini-Spike).
LUMASON and SONOVUE are registered trademarks of Bracco Diagnostics Inc. and its affiliated entities.
All other trademarks and registered trademarks are the property of their respective owners.
IMPORTANT SAFETY INFORMATION | ISOVUE® (Iopamidol Injection) solution
PRODUCT NAME®
IMPORTANT SAFETY INFORMATION
WARNING: Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Praesent mattis eget nunc elementum malesuada. In et nunc lobortis massa maximus ullamcorper nec quis ante. Integer et dolor turpis. Vivamus id semper odio. Integer venenatis viverra elit vel interdum. Nam lectus diam, dictum non erat at, rutrum faucibus tellus. Proin in nulla sagittis, mollis eros eu, iaculis tortor. Nulla non sem scelerisque, congue ante vitae, venenatis urna. Phasellus et viverra leo. Nunc at ultricies felis. Nulla suscipit pulvinar euismod. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Nunc eu erat tincidunt, lacinia arcu eu, maximus lectus.

