Buttons and interactive elements are paired with text to create various web-elements. Colors will adapt to the Product’s styles.
The variety of buttons will give some design flexibility throughout while maintaining consistency.
Tertiary Button
Heading with Icon
Toggle Element
Open Toggle
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Closed Toggle
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Closed Toggle
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.

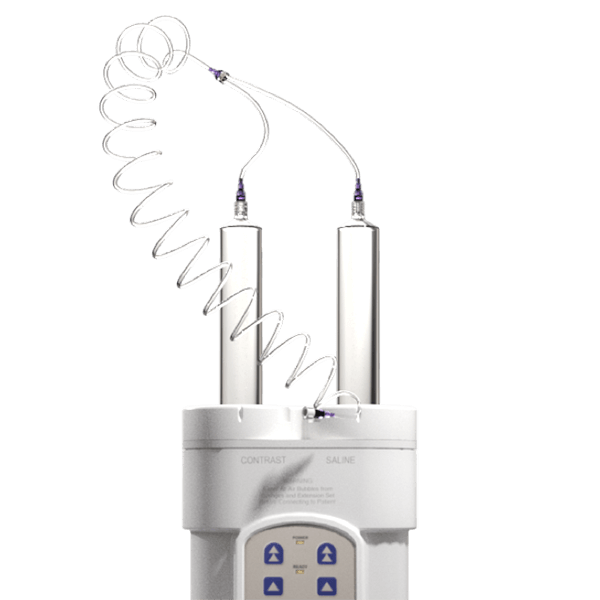

Remote Interface
User-friendly remote interface for easy viewing and programming protocols
Dual Initialize-and-Replace Syringe
Improves clinical workflow and increases productivity
Compact, Rotating Head
Provides more operational flexibility in small spaces
Open Toggle
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Closed Toggle
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Closed Toggle
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Form Styles

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer ullamcorper rhoncus felis, eu viverra urna porta non. Donec at mauris in enim imperdiet iaculis sed quis sem. Duis ipsum lacus, fermentum id odio gravida, maximus dapibus diam. Fusce semper tortor in elit ullamcorper, a lobortis urna elementum. Cras ornare blandit faucibus. Donec tincidunt ante mattis nunc gravida congue. Vivamus euismod, leo eu dictum accumsan, sem odio tristique nisl, non mattis dolor lacus vitae erat. Vestibulum sagittis tincidunt faucibus. Fusce euismod hendrerit viverra. Suspendisse iaculis sodales odio, at accumsan odio consequat ut. Cras porttitor accumsan tortor quis facilisis. Phasellus mollis, mi id aliquam sagittis, turpis neque placerat purus, id volutpat dolor arcu ac tellus. Praesent et dolor ut metus auctor euismod ut et nisi. Quisque lobortis pulvinar enim vitae consectetur. Duis id laoreet nisl, et ultricies eros.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer ullamcorper rhoncus felis, eu viverra urna porta non. Donec at mauris in enim imperdiet iaculis sed quis sem. Duis ipsum lacus, fermentum id odio gravida, maximus dapibus diam. Fusce semper tortor in elit ullamcorper, a lobortis urna elementum. Cras ornare blandit faucibus. Donec tincidunt ante mattis nunc gravida congue. Vivamus euismod, leo eu dictum accumsan, sem odio tristique nisl, non mattis dolor lacus vitae erat. Vestibulum sagittis tincidunt faucibus. Fusce euismod hendrerit viverra. Suspendisse iaculis sodales odio, at accumsan odio consequat ut. Cras porttitor accumsan tortor quis facilisis. Phasellus mollis, mi id aliquam sagittis, turpis neque placerat purus, id volutpat dolor arcu ac tellus. Praesent et dolor ut metus auctor euismod ut et nisi. Quisque lobortis pulvinar enim vitae consectetur. Duis id laoreet nisl, et ultricies eros.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer ullamcorper rhoncus felis, eu viverra urna porta non. Donec at mauris in enim imperdiet iaculis sed quis sem. Duis ipsum lacus, fermentum id odio gravida, maximus dapibus diam. Fusce semper tortor in elit ullamcorper, a lobortis urna elementum. Cras ornare blandit faucibus. Donec tincidunt ante mattis nunc gravida congue. Vivamus euismod, leo eu dictum accumsan, sem odio tristique nisl, non mattis dolor lacus vitae erat. Vestibulum sagittis tincidunt faucibus. Fusce euismod hendrerit viverra. Suspendisse iaculis sodales odio, at accumsan odio consequat ut. Cras porttitor accumsan tortor quis facilisis. Phasellus mollis, mi id aliquam sagittis, turpis neque placerat purus, id volutpat dolor arcu ac tellus. Praesent et dolor ut metus auctor euismod ut et nisi. Quisque lobortis pulvinar enim vitae consectetur. Duis id laoreet nisl, et ultricies eros.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer ullamcorper rhoncus felis, eu viverra urna porta non. Donec at mauris in enim imperdiet iaculis sed quis sem. Duis ipsum lacus, fermentum id odio gravida, maximus dapibus diam. Fusce semper tortor in elit ullamcorper, a lobortis urna elementum. Cras ornare blandit faucibus. Donec tincidunt ante mattis nunc gravida congue. Vivamus euismod, leo eu dictum accumsan, sem odio tristique nisl, non mattis dolor lacus vitae erat. Vestibulum sagittis tincidunt faucibus. Fusce euismod hendrerit viverra. Suspendisse iaculis sodales odio, at accumsan odio consequat ut. Cras porttitor accumsan tortor quis facilisis. Phasellus mollis, mi id aliquam sagittis, turpis neque placerat purus, id volutpat dolor arcu ac tellus. Praesent et dolor ut metus auctor euismod ut et nisi. Quisque lobortis pulvinar enim vitae consectetur. Duis id laoreet nisl, et ultricies eros.
Signup Integrated Form Element
Image Slider Element
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
PRODUCT NAME®
IMPORTANT SAFETY INFORMATION
WARNING: Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Praesent mattis eget nunc elementum malesuada. In et nunc lobortis massa maximus ullamcorper nec quis ante. Integer et dolor turpis. Vivamus id semper odio. Integer venenatis viverra elit vel interdum. Nam lectus diam, dictum non erat at, rutrum faucibus tellus. Proin in nulla sagittis, mollis eros eu, iaculis tortor. Nulla non sem scelerisque, congue ante vitae, venenatis urna. Phasellus et viverra leo. Nunc at ultricies felis. Nulla suscipit pulvinar euismod. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Nunc eu erat tincidunt, lacinia arcu eu, maximus lectus.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
PRODUCT NAME®
IMPORTANT SAFETY INFORMATION
WARNING: Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Praesent mattis eget nunc elementum malesuada. In et nunc lobortis massa maximus ullamcorper nec quis ante. Integer et dolor turpis. Vivamus id semper odio. Integer venenatis viverra elit vel interdum. Nam lectus diam, dictum non erat at, rutrum faucibus tellus. Proin in nulla sagittis, mollis eros eu, iaculis tortor. Nulla non sem scelerisque, congue ante vitae, venenatis urna. Phasellus et viverra leo. Nunc at ultricies felis. Nulla suscipit pulvinar euismod. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Nunc eu erat tincidunt, lacinia arcu eu, maximus lectus.
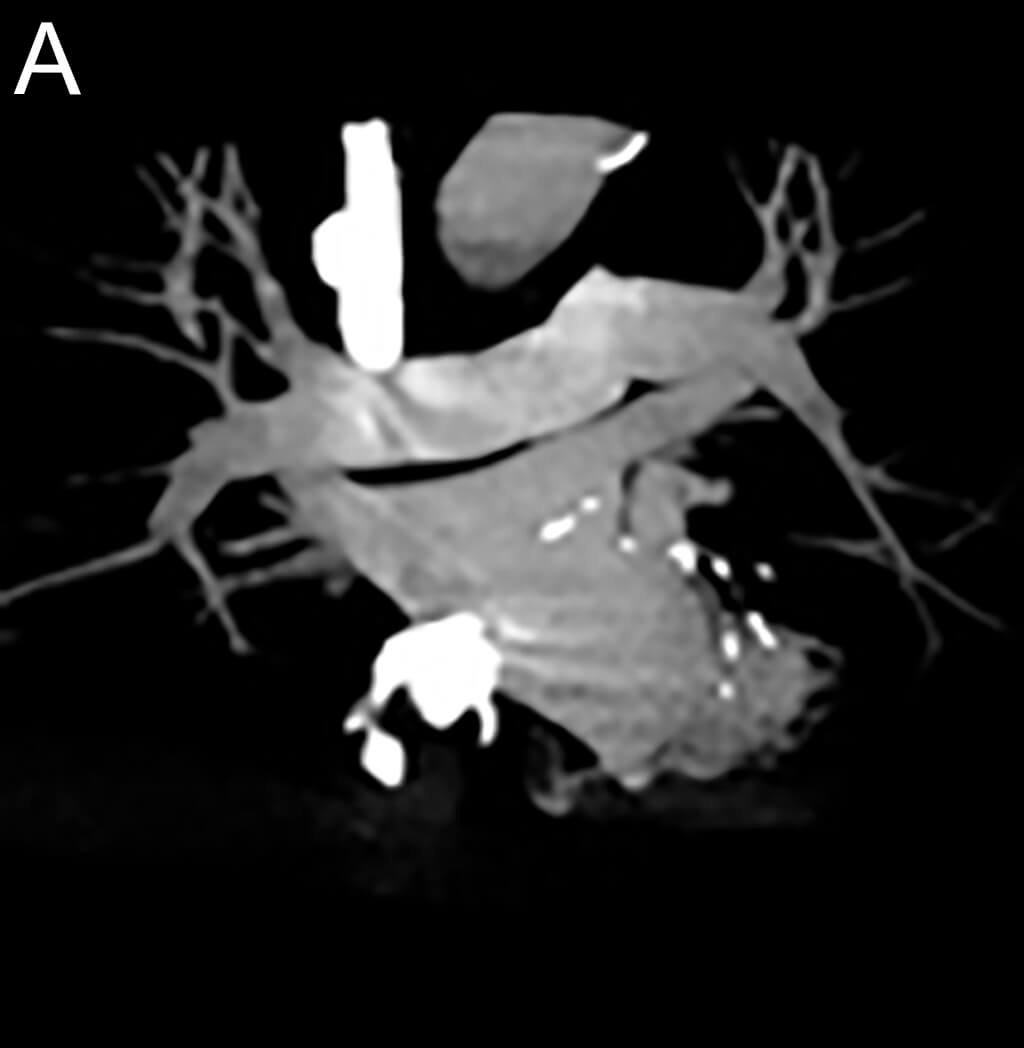
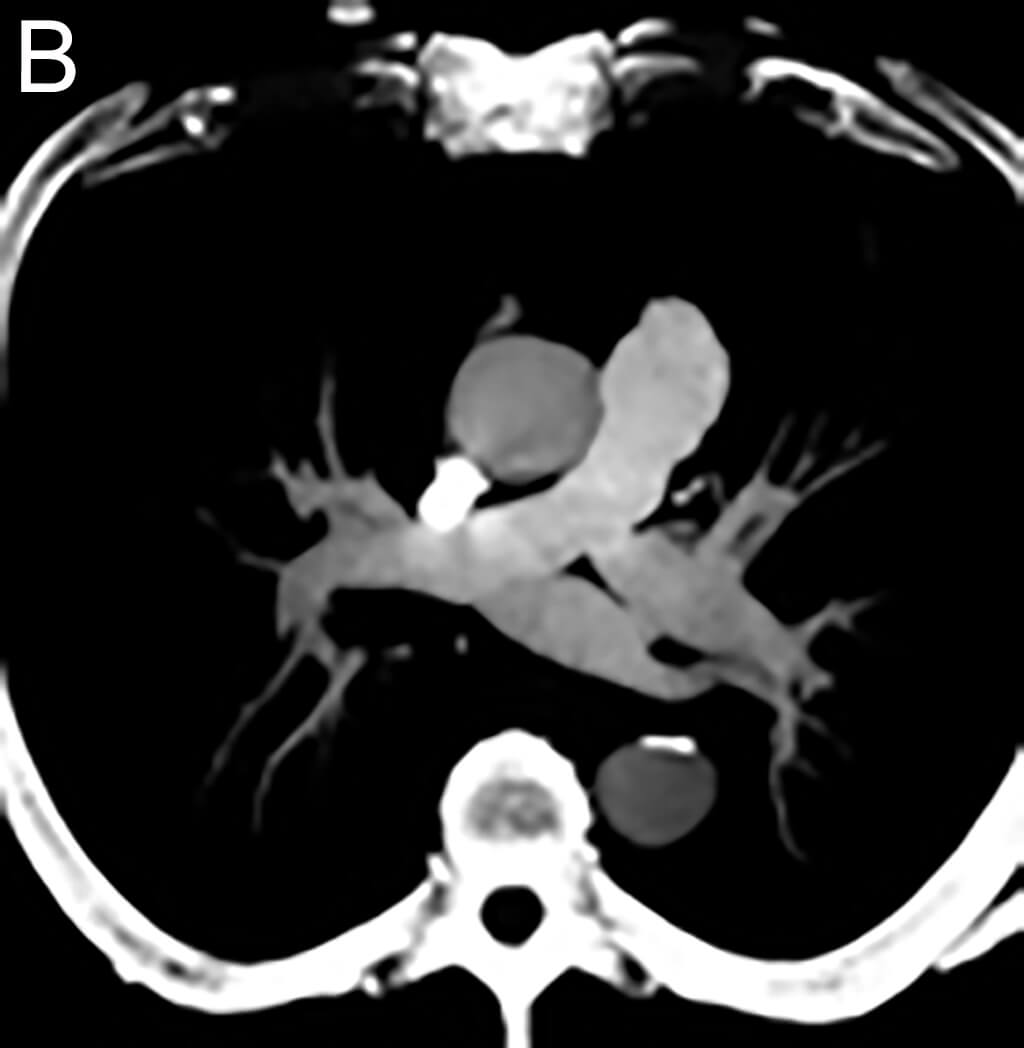
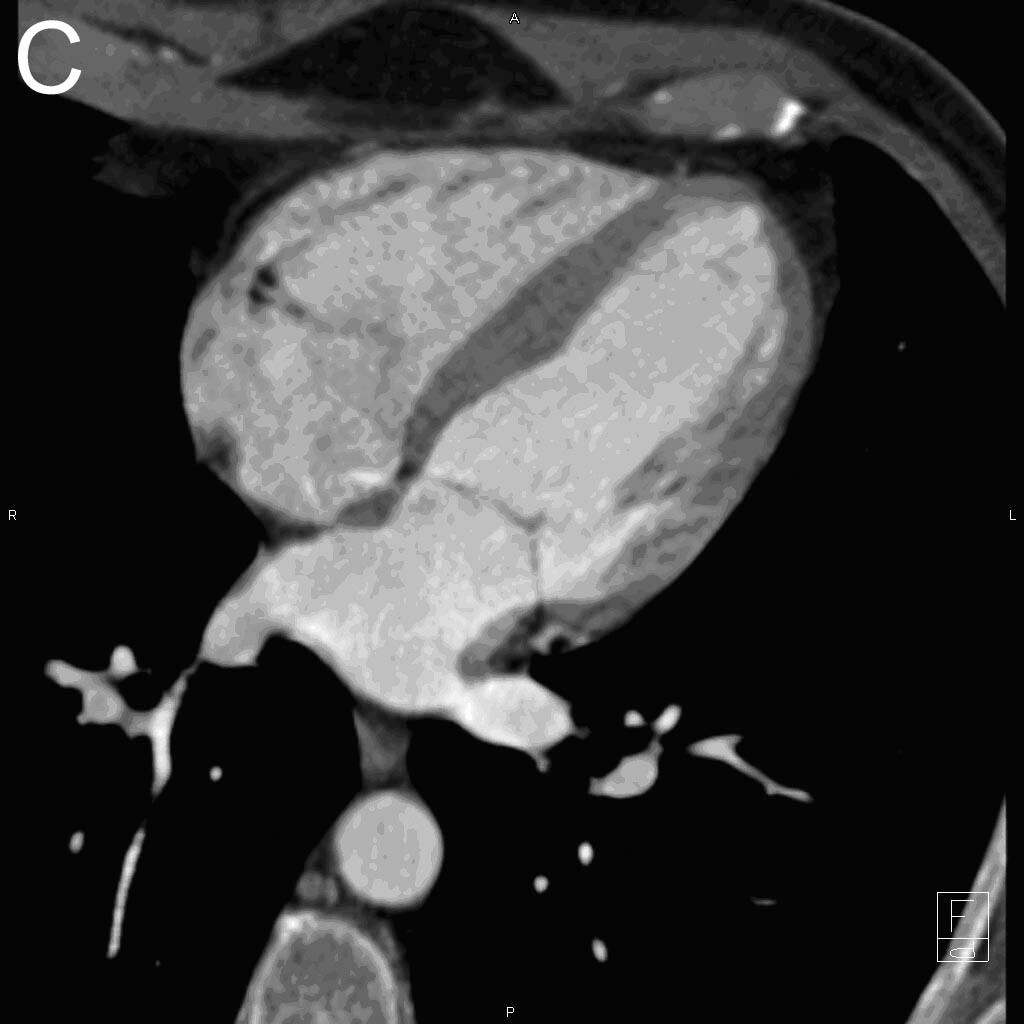
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes administration
- Always have resuscitation equipment and trained personnel readily available
Contraindications
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is contraindicated in patients with known or suspected hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG).
Warnings
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including LUMASON. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to LUMASON administration and monitor all patients for acute reactions.
Post-marketing hypersensitivity reactions, including serious hypersensitivity reactions, have been observed during use or shortly following LUMASON administration. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. LUMASON contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG.
Systemic embolization may occur in patients with cardiac shunts. Assess patients with cardiac shunts for embolic phenomena following LUMASON administration.
There is a risk of ventricular arrhythmia related to high mechanical index in patients administered LUMASON. LUMASON is not recommended for use at mechanical indices greater than 0.8.
The most common adverse reactions (incidence ≥ 0.5%) are headache (1%) and nausea (0.5%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for LUMASON ultrasound contrast agent, including BOXED WARNING on Serious Cardiopulmonary Reactions.
LUMASON is manufactured for Bracco Diagnostics Inc., Monroe Township, NJ 08831 by Bracco Suisse S.A., Plan-les-Ouates Geneve, Switzerland (LUMASON lyophilized powder vial-25 mg lipid-type A/60.7 sulfur hexafluoride gas); Vetter Pharma-Fertigung GmbH & Co. KG, 88212 Ravensburg, Germany (Sodium Chloride 0.9% Injection, USP); B. Braun Melsungen AG, 34212 Melsungen, Germany (Mini-Spike).
LUMASON and SONOVUE are registered trademarks of Bracco Diagnostics Inc. and its affiliated entities.
All other trademarks and registered trademarks are the property of their respective owners.
IMPORTANT SAFETY INFORMATION | ISOVUE® (Iopamidol Injection) solution
PRODUCT NAME®
IMPORTANT SAFETY INFORMATION
WARNING: Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Praesent mattis eget nunc elementum malesuada. In et nunc lobortis massa maximus ullamcorper nec quis ante. Integer et dolor turpis. Vivamus id semper odio. Integer venenatis viverra elit vel interdum. Nam lectus diam, dictum non erat at, rutrum faucibus tellus. Proin in nulla sagittis, mollis eros eu, iaculis tortor. Nulla non sem scelerisque, congue ante vitae, venenatis urna. Phasellus et viverra leo. Nunc at ultricies felis. Nulla suscipit pulvinar euismod. Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas. Nunc eu erat tincidunt, lacinia arcu eu, maximus lectus.